Information on Benzene (C6H6)
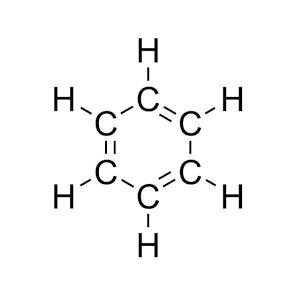
Molecular Structure
Benzene (C6H6) is a cyclic hydrocarbon made of six carbon atoms and six hydrogen atoms. The six carbon atoms are arranged in a hexagonal ring, with one hydrogen atom bonded to each carbon atom. What makes benzene different from others is its resonance structure: instead of alternating single and double bonds, the six π (pi) electrons are delocalized evenly across the entire ring. This delocalization gives benzene its characteristic aromatic stability, making it significantly less reactive than regular alkenes. Because of this stable aromatic system, benzene opposes additional reactions that break the ring, and instead it goes through substitution reactions, where one hydrogen is replaced by another atom or group.

Chemical & Physical Properties
Benzene is a colourless, flammable liquid with a sweet odour. It has a boiling point of 80.1°C and a melting point of 5.5°C. Since it is nonpolar, benzene does not mix well with water but dissolves easily in oils, fats, and other organic solvents. Its volatility allows it to evaporate rapidly into the air, making inhalation the most frequent way people are exposed. Benzene doesn't undergo additional reactions like typical alkenes, but instead, it undergoes electrophilic substitution reactions. Benzene's stability comes from its aromatic ring of delocalized electrons, which makes it resistant to breaking down and slow to degrade in the environment.
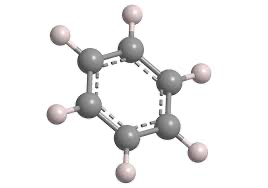
Functional Group
The functional group of benzene (C6H6)is aromatic ring. In larger compounds, it's called a phenyl group (-C6H5). The aromatic ring is the source of both its chemical stability and its unique reactivity. Benzene belongs to the aromatic hydrocarbons family. The aromatic hydrocarbon family consists of organic compounds containing one or more stable, planar ring structures called benzene rings. Unlike alcohols (-OH), carboxylic acids (-COOH), or ketones (C=O), benzenes defining feature is its ring of six carbons with delocalized π-electrons. This aromatic functional group gives benzene unusual resonance energy and causes it to undergo substitution reactions instead of addition reactions.
Create Your Own Website With Webador